Gene Therapy For Sickle Cell Disease 2025. By american college of physicians. December 8, 2025, 10:00 am est.
Identify the benefits and limitations of currently available therapies for sickle cell disease, including newly approved agents. Get the latest updates frommit technology review.
The two gene therapy treatments for sickle cell disease recently approved by the fda, called casgevy and lyfgenia, cost $2.2 million and $3.1 million per patient,.
The findings from a new clinical trial, published august 31, add to the body of evidence supporting gene therapy as a treatment for sickle cell disease, which primarily impacts.
NIH researchers create new viral vector for improved gene therapy in, The university of chicago medicine comer children’s hospital will be among the first in the country to offer gene therapy for sickle cell. The fda on friday also approved a second treatment for sickle cell disease, called lyfgenia, a gene therapy from drugmaker bluebird bio.
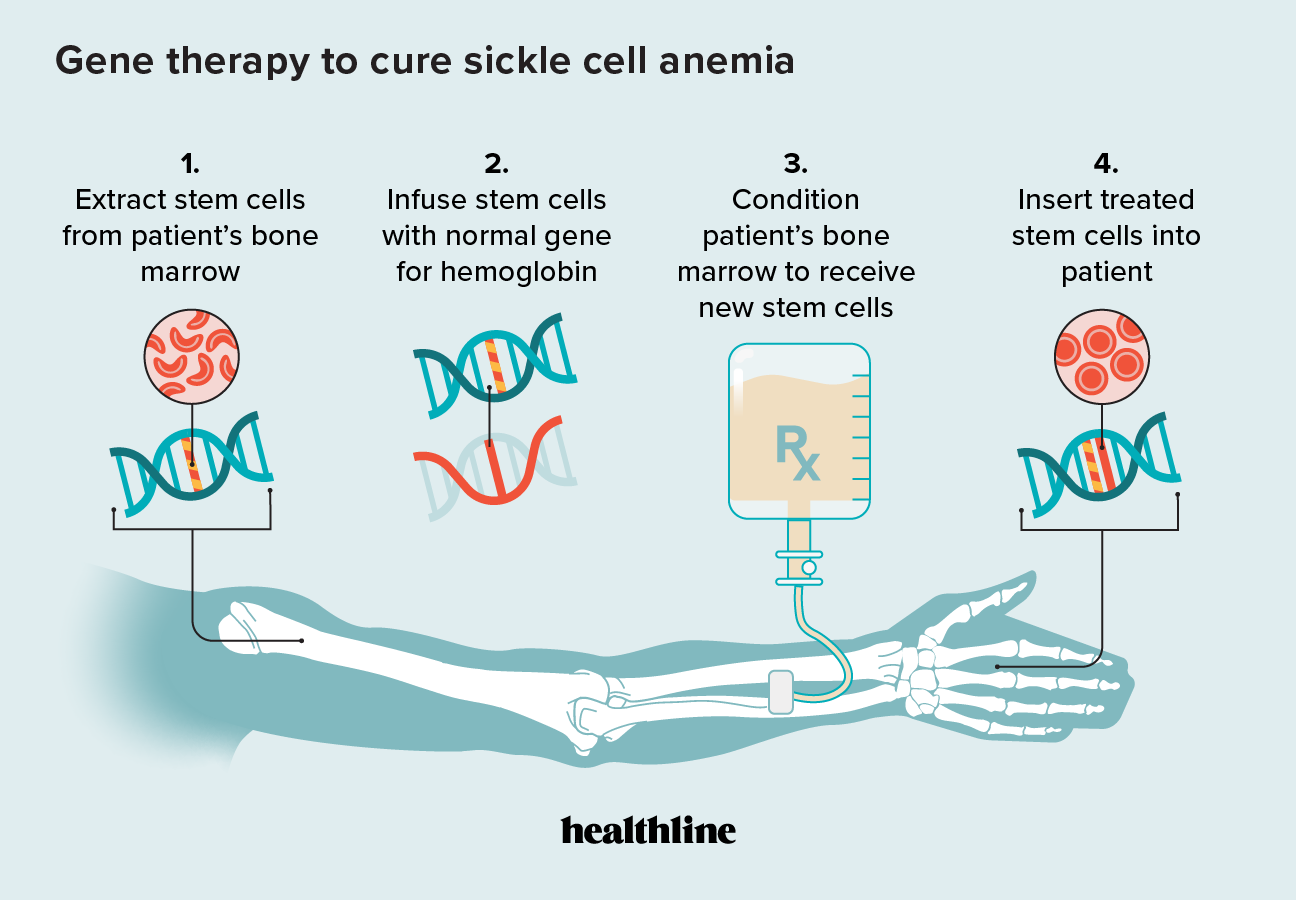
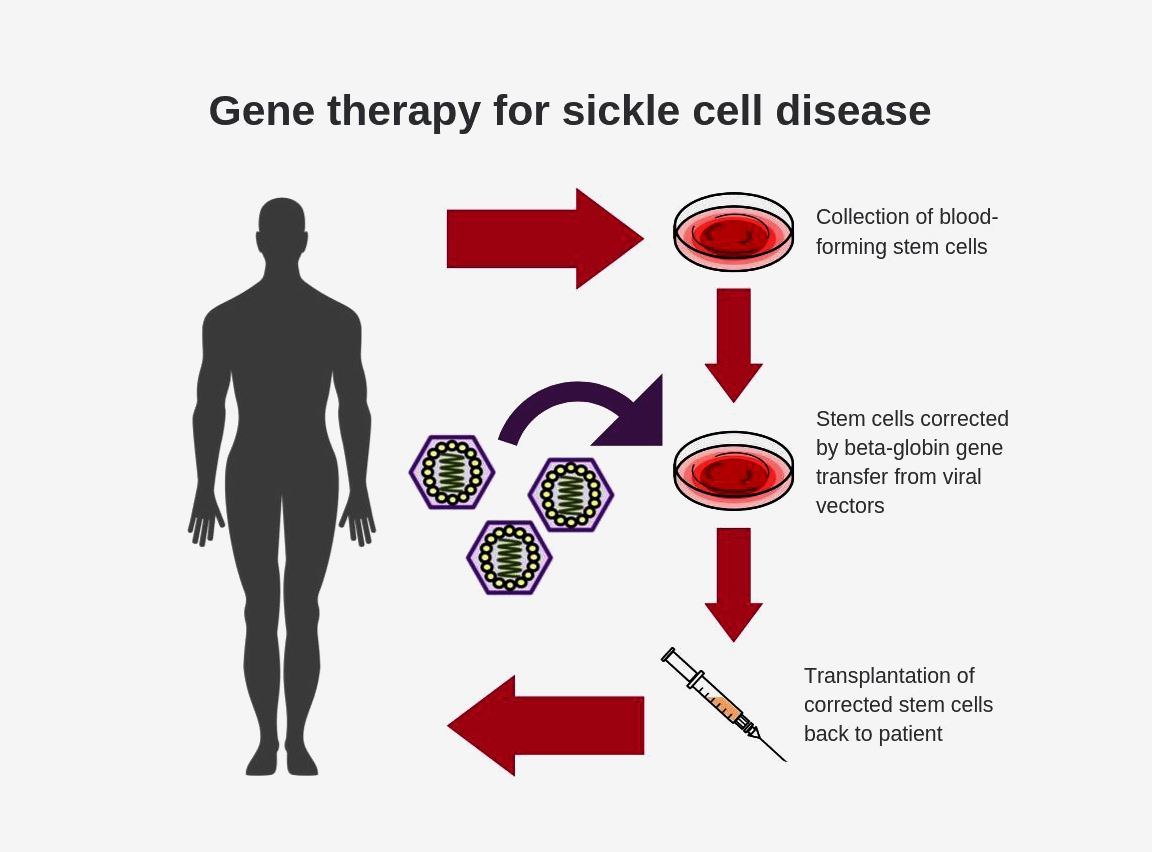
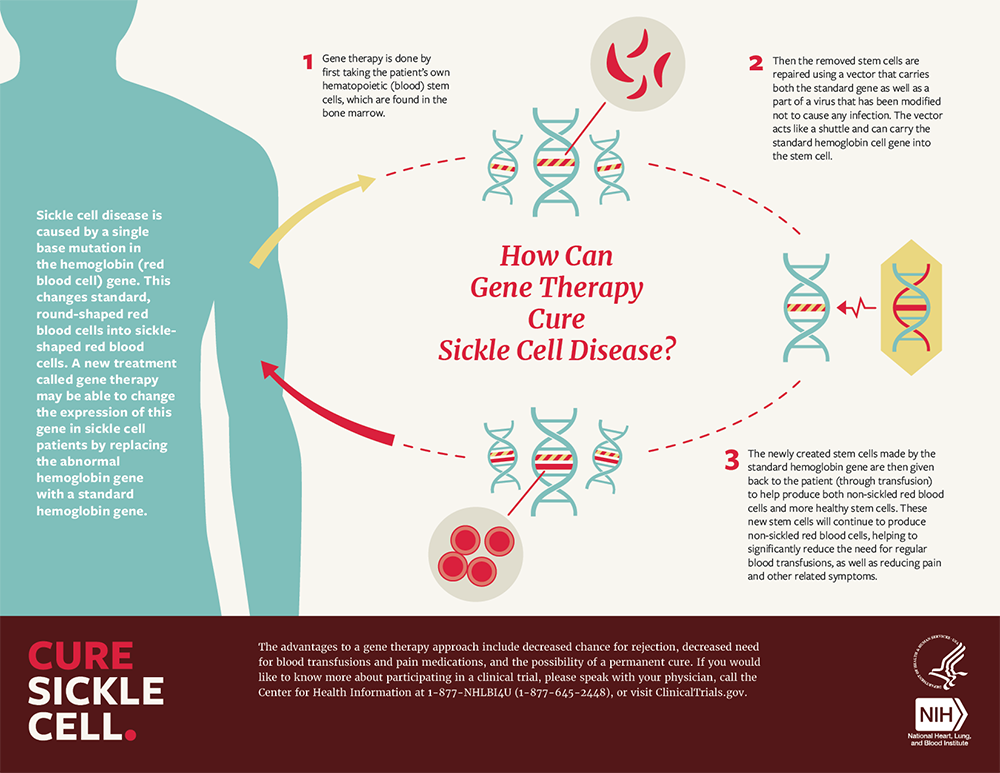
Gene Editing for Sickle Cell Disease Succeeded Bioinformatics Hub, The university of chicago medicine comer children’s hospital will be among the first in the country to offer gene therapy for sickle cell. Gene therapy is an emerging treatment for sickle cell disease that works by replacing a defective gene with a healthy gene, allowing the body to produce normal.
Sickle Cell Anemia and Gene Therapy How It Works, The findings from a new clinical trial, published august 31, add to the body of evidence supporting gene therapy as a treatment for sickle cell disease, which primarily impacts. The university of chicago medicine comer children’s hospital will be among the first in the country to offer gene therapy for sickle cell.
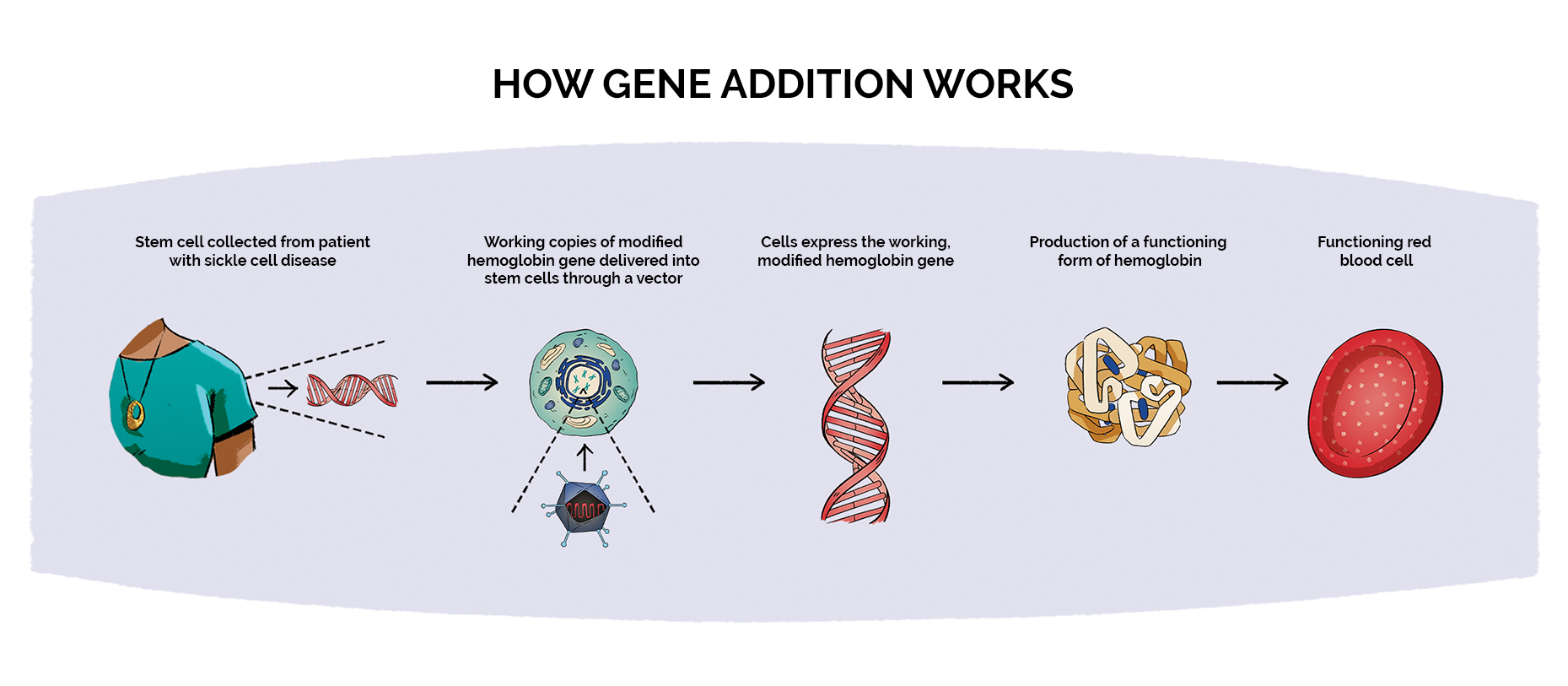
50 Shocking Statistics on Sickle Cell Anemia You Must Know 2025, These are the first treatments of their kind available to individuals with scd. The findings from a new clinical trial, published august 31, add to the body of evidence supporting gene therapy as a treatment for sickle cell disease, which primarily impacts.
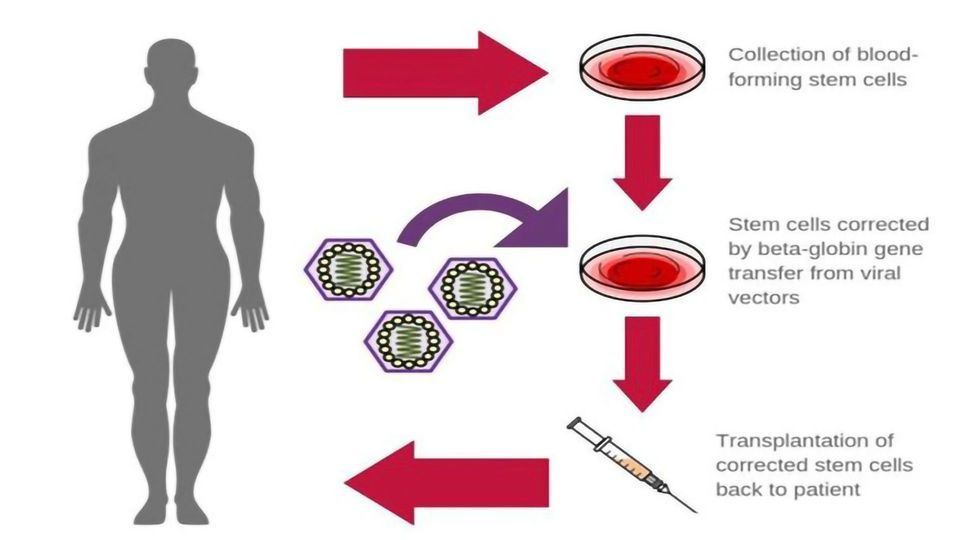
Gene Therapy in Sickle Cell Disease, The university of chicago medicine comer children’s hospital will be among the first in the country to offer gene therapy for sickle cell. Identify the benefits and limitations of currently available therapies for sickle cell disease, including newly approved agents.
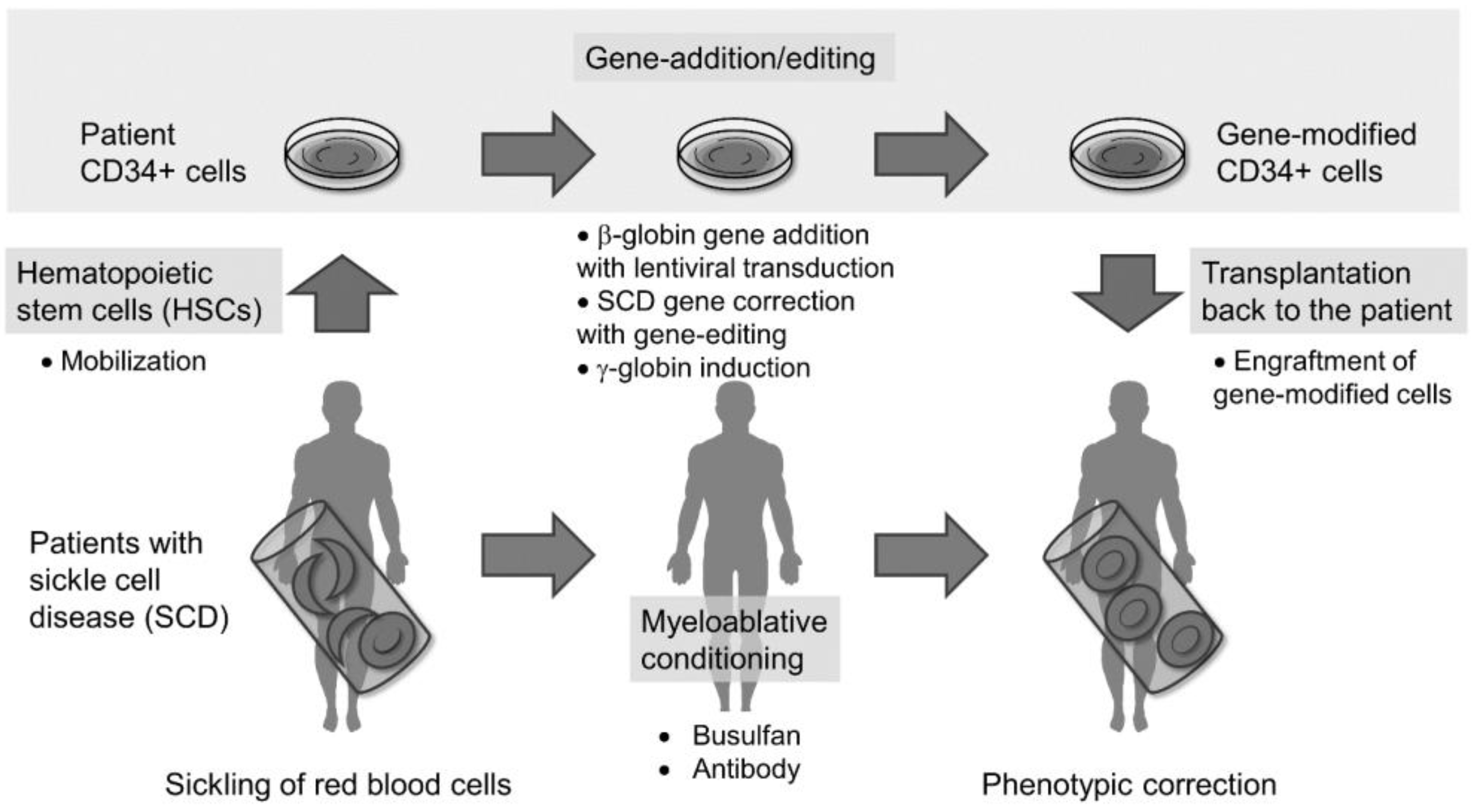
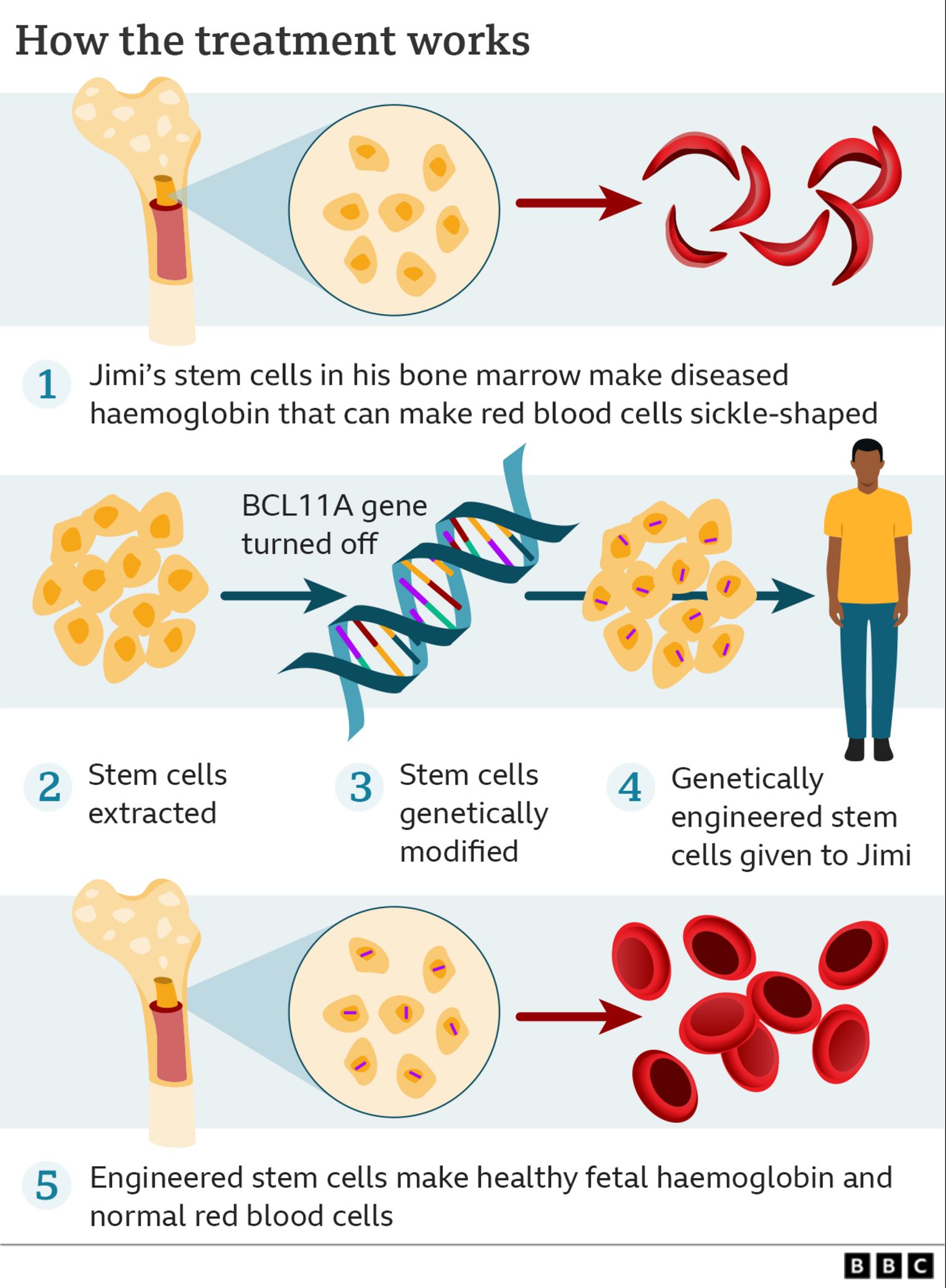
UK Approves Gene Therapy for Sickle Cell Disease, Gene therapy is an emerging treatment for sickle cell disease that works by replacing a defective gene with a healthy gene, allowing the body to produce normal. The university of chicago medicine comer children’s hospital will be among the first in the country to offer gene therapy for sickle cell.

Cells Free FullText Hematopoietic Stem Cell GeneAddition/Editing, The fda on friday also approved a second treatment for sickle cell disease, called lyfgenia, a gene therapy from drugmaker bluebird bio. Two products were approved in early december:
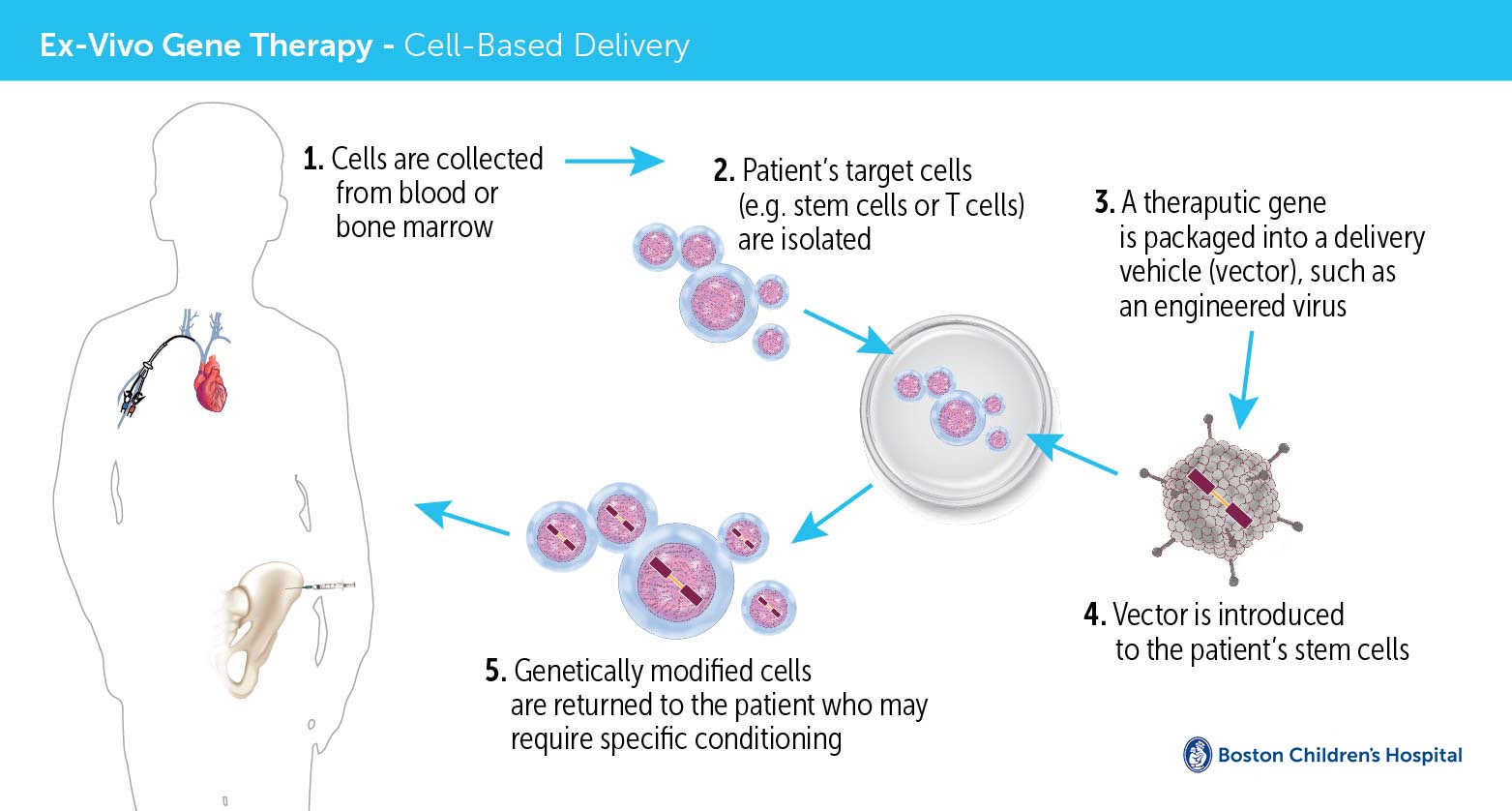
Positive Results Seen With Voxelotor in Pediatric Patients With Sickle, Two products were approved in early december: The two gene therapy treatments for sickle cell disease recently approved by the fda, called casgevy and lyfgenia, cost $2.2 million and $3.1 million per patient,.
Moving gene therapy into high gear Boston Children's Answers, By american college of physicians. Fda clears sickle cell drug to treat another blood disorder.
Experimental gene therapy reverses sickle cell disease for three years, Normal blood cells next to. Food and drug administration (fda).
Gene therapy is an emerging treatment for sickle cell disease that works by replacing a defective gene with a healthy gene, allowing the body to produce normal.